FACULTY OF HEALTH SCIENCES
MLT 428
HISTOLOGICAL TECHNIQUES
OCT 2021 - FEB 2022
LABORATORY REPORT
TITLE: ROUTINE PROCEDURE IN HISTOPATHOLOGY LAB
Prepared by: Student's ID:
Student's name: 2020498516
IEESYA ATIFAH BINTI MOHD FAUZI
NOR ALIAH SYAHIRAH BT MOHD HADZIR 2020498732
NUR ALYAA QALISHA BINTI MOHAMMAD SAID 2020897946
NUR MAISARAH BINTI MOHAMAD 2020853802
WAN ANISSA BINTI WAN MOHD ZAKI 2020498894
LECTURERS:
MADAM HARTINI YUSOF
DR. AYUNIE BINTI ZULKEPLI
WORKFLOW1.0 & the laboratory
equipments.
MLT 428 HISTOLOGICAL TECHNIQUES
TISSUE PREPARATION TISSUE PROCESSING TISSUE EMBEDDING
Trimming or dissection of selected Eliminate water from cells and To position and pour paraffin wax
tissue specimens fixed with fixative replace it with a solidifying into the specific mould holding
solution for preparing proper tissue media the processed tissue specimen,
blocks. resulting in a paraffin-embedded
tissue block.
AUTOMATED TISSUE PROCESSOR:
SHANDON CITADEL 1000 TISSUE SECTIONING
To dehydrate tissue, and Trimming: Exposing an
replace the water with a appropriate amount of tissue and
support medium that gives assuring the top and bottom of
it sufficient rigidity. the block are parallel and
horizontal to the blade's edge
when sectioning
Sectioning: To make thin slices for
staining on microscope slides
TISSUE EMBEDDING CENTRE: SLEE MAINZ MPS/C TISSUE STAINING
To produce paraffin block of tissue by embedding the tissue using
To differentiate the nucleus and
melted paraffin or waxes that provides strong external support cytoplasm by contrasting colors
during sectioning of tissue when its cooled.
by Haematoxylin and Eosin
Staining.
MICROTOME: SLIDE MOUNTING &
To permits LABELLING
extremely
thin slices To mount the sample with a
coverslip using a mounting
of medium for protecting the
material,
known as sample physically.
sections,
to be cut. FROZEN SECTION
To prepare slide for rapid
tissue interpretation under
microscope using the cryostat.
SLEE Mainz CUT5062
1.0.1
INTRODUCTION
MLT 428 HISTOLOGICAL TECHNIQUE
In the 1700s, the word “tissue” was introduced by Marie-François Xavier Bichat to the
medical dictionary and the composition of organs from tissues was then clearly
understood. The study was then continued by J.J Lister to complete the study by
improving the microscope system and got to work on histology analysis. Histological
staining also developed in the 1700s. However, the first use of hematoxylin was in 1863
by Wilhelm von Waldeyer as a nuclear stain and it is widely used now in tissue staining.
Histology, an alias to microscopic anatomy or microanatomy, is a branch of biological
studies of tissues and their respective structure. It helps hospital laboratories to identify
the disease processes that affect tissues depending on their own classes and the disease
itself. The study also allows laboratories to check for abnormalities in cells that indicate
the patient's illness's true aetiology by conducting specific procedures.
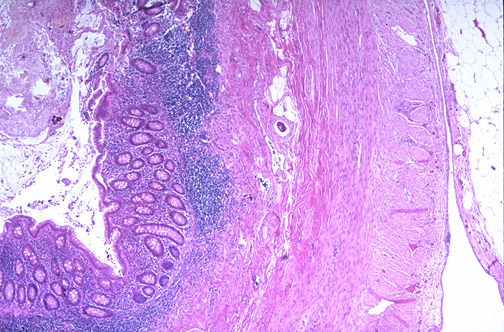
Diagnosis can be made by looking at a little piece of tissue from different organs. As in
this lab report, we mainly focus on the tonsil, appendix and uterus parts in the
laboratory. To examine tissue features and microscopic structures of cells, a variety of
approaches had applied. Histological staining is a methodology in preparing sample
tissues for examination under the microscope by staining them with histological stains.
The stain used in this experiment is a basic dye, Hematoxylin and acidic dye, Eosin.
Mostly known as H&E stain. The stained microscope sections will guide the hospital
laboratories by highlighting the anatomical structure of the cells that appear within the
tissues.
Tissueblockclamped before Asideviewofuterusparaffin Aplanviewofuterusparaffin Aplanviewof appendix Acompleteslideofappendix
trimming block block paraffinblock
NUR ALYAA QALISHA BINTI MOHAMMAD SAID
WAN ANISSA BINTI WAN MOHD ZAKI
1.1Prepared by: NUR MAISARAH BINTI MOHAMAD
TISSUE PREPARATION
tissue grossing and fixation
MATERIAL REAGENT APPARATUS
Human tissue specimens such as 10% of commercially prepared formalin 1. Mask
2. Gloves
appendix and tonsil fixed with RESULTS 3. Lab coat/protective clothing
4. Medisheet / Grossing paper
commercially prepared 10% Tissue blocks 5. Dissecting board
formalin (prefered to use plastic board)
6. Forceps
PRINCIPLE 7. Scalpel handle
8. Scalpel blades
GROSSING 9. Tissue cassettes
An examination and dissection process of tissue that was an initial and 10. Containers with appropriate lids
essential steps in the histology lab to obtain an accurate diagnosis of 11. Ruler
patients, macroscopically and microscopically. 12. Pencil
FIXATION: EQUIPMENT
Fixative solution which is a 10% of commercially prepared formalin
preserved the tissues permanently in their natural state as possible by 1.Ducted Fume Hood: Iryas
stopping enzyme activity, killing microorganisms, hardened the tissues
and fixed all components of tissues. It also enhanced the staining of
tissue steps.
PROBLEM
PROCEDURE:
1. Thickness of tissue that was more
1. A complete PPE (personal protective equipment) was worn properly to than 4mm, often have an uneven
avoid the risk of any potential hazard. surface of tissues after soaking the
tissue in the fixative solution.
2.Cassettes were labeled with name/student ID number, date and type of
specimen by using a pencil. 2. Some of the tissue parts was not in
the same layer affect the entire
3.Grossing workstation in the Ducted Fume Hood were prepared with process to get a perfect sectioning of
instruments that can be readily used for tissue grossing. The fume hood tissue.
light and suction vent switches were turned on. The workstation could be
clearly seen and well ventilated to prevent inhalation of formaldehyde 3. The tissue block surface was not
fumes. smooth due to trimming technique.
4.Only one specimen container that was opened at one time to avoid mix- SOLUTION
ups of specimens.
1. A uniform and thin slices of tissue that
5.Grossing or trimming of tissues with proper orientation has been done have 3-4 mm thickness prepared by
using scalpel and forceps according to accepted protocols for the using a ruler.
laboratory. It is performed one at a time and ensured the trimmed tissues
were approximately 3-4mm thick by using the ruler. 2. Excess of tissues were removed if it is not
in the same layer of the entire tissue.
6.Macroscopic detailed conditions of the tissues such as its color, unusual
tissue surface or pigmentation and apparent abnormalities were 3. Cut the tissue in one way motion. Avoid
described. to cut the tissue in forward and backward
motion.
7. The trimmed tissue was transferred into a labeled cassette by using a
forceps. CONCLUSION
8.Cassettes containing trimmed tissue were immersed in 10% of Grossing and fixation main purpose to
commercially prepared formalin containers for 6 to 24 hours. get an accurate and diagnostically
dissection of preserved tissue with
9..Formalin waste was discarded into formalin waste container proper orientation was obtained .
In tissue preparation, it is also important
to always replace the fixative formalin
solution when the specimen contain high
percentage of blood.
1.2 Prepared by: NOR ALIAH SYAHIRAH BT MOHD HADZIR
TISSUE PROCESSING
PRINCIPLE: EQUIPMENT
Automated Tissue Processor
1. DEHYDRATION (Model: Shandon Citadel 1000)
The process where water is removed from the tissue including the
unbound fixative REAGENTS
50% Alcohol (2L)
2. CLEARING 70% Alcohol (2L)
Displaces the dehydrating solution, allowing the infiltration 80% Alcohol (2L)
medium to reach the tissue component. 95% Alcohol (2L)
Alcohol + Xylene
3. INFILTRATION
RESULT
Paraffin wax is infiltrated or injected into tissue sections to stabilise
the tissue and allow thin slices to be cut. Processed Tissue
PROCEDURE: TISSUE PROCESSING CYCLE
1.All the processing reagents were
prepared 10% Buffered Formalin 2:00
2.All the prepared processing reagents 50% Alcohol 1:00
were placed in the corresponding 70% Alcohol 1:00
container 80% Alcohol 1:00
3.All the cassettes containing tissue 95% Alcohol 1:00
specimens were transferred into the tissue Absolute Alcohol 2:00
processor organiser basket using forceps Absolute Alcohol 2:00
4.The tissue processor organizer baskets Mixture of Absolute Alcohol and Xylene 1:00
were placed into the basket hanger. Xylene 2:00
5.The lid of the tissue processor basket was Xylene 2:00
placed on top of the organiser basket Paraffin Wax 3:00
6.The basket was firmly inserted into the Paraffin Wax 3:00
operating head slot Total 21:00
7.Using the hand-held controller, each step
for the processing time is properly PROBLEM CAUSES
programmed
8.To begin the tissue processing cycle, press Tissue feels soft during Paraffin may be
“Auto Start” button and close the embedding. saturated with xylene
operating door. Tissue was processes
9.Tissue Processing Cycle began. Tissue bounce out from for too short
block during tissue The processing
Tissue processing is a very important step that must sectioning. reagents may have
be closely monitored as it takes longer to process the saturated with water
tissue, any mistakes will cause the tissues to change,
necessitating reprocessing. Water remaining in the
The tissue should not be underprocessed or tissue.
overprocessed, as this will affect the tissue details.
CONCLUSION It's critical that the tissues are treated properly and Replace reagents and reprocess
rendered ready for the next phases.
SOLUTION tissue according to the correct
protocol.
1.3 Prepared by: NUR ALYAA QALISHA BINTI MOHAMMAD SAID
TISSUE EMBEDDING
PRINCIPLE: MATERIAL:
The embedding processing concept is to properly and 1. Processed tissue
precisely position a histology specimen into a block of REAGENTS:
paraffin wax. This is to ensure and allow the tissue sample 1.Paraffin wax (SAKURA Tissue-Tek
to be supported and held in place so that precision cutting
of thin sections for histological diagnostic purposes can be Paraform & Surgipath Paraplast Plus)
made for further process APPARATUS:
PROCEDURE: 1. Forceps
2.Steel block mould
1.Processed tissue cassettes were removed from the 3. Scraper
tissue processing machine. 4. Gloves
5.Tissue paper
2.The tissue cassettes were transferred into pre warmer 6. Masks
unit of the tissue embedding centre to be pre- EQUIPMENT:
warmed. 1.Paraffin Wax Tissue Embedding Centre:
SLEE Mainz MPS/C / MPS/P / MPS/W
3.Cassette was opened to view the tissue sample and an
appropriate mould is chosen based on the tissue RESULT
sample size.
Figure 1 : Good paraffin-
4.A small amount of molten paraffin was poured into embedded tissue block of
the mould. appendix
5.With warm forceps, processed tissue was transferred Figure 2: Slight crack along
into the mould and arranged the tissue according to the edges of paraffin-
the sectioning side. This side of the mould is facing embedded tissue block
down
PROBLEM SOLUTION
6.Mould transferred into a cold plate. The tissue was
firmly and gently pressed flat with a pair of warm and - Presence of bubble Wax is dispensed slowly
cleaned forceps.
when dispensing wax into and bubbles is eliminated
7.Paraffin was poured into the mould fully until covered
the face of the cassette. the mould. before cooling the wax.
CONCLUSION 8. The labelled paper was placed on top of the c1a. ssette. - Position of the tissue Tissue specimen is gently
9.Immediately cooled down by placing the mould on the specimen was not centered anchored at the center of
and slightly slanted. the mould when cooling
cryo console. the wax. The flat side is
10.Solidified paraffin wax separated from the mould to - Tissue is incorrectly position downward.
orientated .
form a paraffin tissue block.
11.Excess wax from the paraffin tissue block is removed - Presence of crack at the Gently tapped the mould
side of the cooled block on the palm or by using
by using a scalpel. due to intense slamming scalpel to clean excess
on the table for removal. wax and the side of the
When paraffin wax was in liquid, it covers the tissue and block is picked carefully.
solidifies quickly when cooled. The medium was
penetrated into the tissue, providing a matrix and
avoiding tissue morphology alteration during microtome.
The orientation of the specimen during embedding is
significant for demonstrating appropriate morphology
and more uniform embedding.
1.4 Prepared by: NUR ALYAA QALISHA BINTI MOHAMMAD SAID
TISSUE SECTIONING
MATERIAL: PRINCIPLE:
1.Paraffin tissue blocks
The tissue sectioning process is a technique
APPARATUS: in cutting a paraffin-embedded or frozen
1.High profile microtome slides tissue into a thin slice in a clean and
2.Clean frosted end glass slides consistent manner. The tissue sections are
3.Clean brush thin slices that are later placed on a glass
4.Applicator sticks slide. The paraffin sectioning and frozen
5. Forceps sectioning are two basic types of sectioning.
6.Slide racks
7.Pasteur pipettes PROCEDURE: Date: 16 October
8.Clean gauze
9.Tissue paper 1.The tissue floatation bath temperature is set at 42°C .
2.The slide is labelled with name, student identification
10. Pencils
EQUIPMENT: number, type of specimen and date.
3.The paraffin-embedded tissue block is placed
1.Rotary microtome: SLEE Mainz CUT5062
2.Tissue floatation bath: XH-1001, Thermo vertically or horizontally and trimmed at 5-10 micron
until a complete tissue section appears on the tissue
Scientific 3120058 ribbon.
3. Freezer 4.The trimmed paraffin block was cooled by putting it
4. Oven into the freezer for 5 minutes before sectioned.
5.Tissue block was ensured fixed in position and new
RREESSUULLTT clean or unused area of microtome blade is used for
sectioning purposes.
Figure 3: Thin section of Figure 4: Ribbon-like thin 6.Sectioning of the paraffin tissue block started to
appendix on glass slide tissue sectioning produce a ribbon section.
7.By using applicator sticks or forceps, the ribbon section
PROBLEM SOLUTION was pulled from the blade.
8.The ribbon sections were transferred into the water
- Visible lines can be The microtome blade is bath.
seen on the ribbon when moved to the untouched 9.The ribbons were laid on the water bath carefully to
sectioning due to a blunt area or changed into a avoid the formation of wrinkles or bubbles on the
blade from trimming. new blade and returned tissue.
to sectioning. 10.The best section from the ribbon sections was selected
and separated gently by using the applicator stick.
- The sectioning is seen to Refreeze the paraffin 11.Residual wrinkled or bubble forming tissue sections
be creased and pressed block for another 5 were removed from the floatation by using a Pasteur
together due to warm minutes or the paraffin pipette.
block or rapid cutting. block is slowly and evenly 12.Floating out (fishing) was performed by using a glass
cut. slide on the selected sections. The applicator stick is
used to prevent the tissue section from moving.
- The sectioning failed to The angle of the blade is 13.The glass slide was gently raised above the water
form a ribbon due to adjusted to desired angle surface and the glass slide containing the tissue
incorrect or inappropriate The block was cooled section is placed onto the slide rack.
angle of the blade. again 14.A glass slide was placed on the slide rack at room
temperature to remove water from the slides.
15.Dried slides were placed into the oven at 60°C for
dewaxing purposes before continuing with staining.
- Water trapped under the Making sure the slide is CONCLUSION The tissue section will be needed to be cut at 3 to 4 microns
thin section after fishing at 45° angle and with no wrinkles, folds, or air bubbles under the sections.
out from water bath due to fished out at the same Tissue thickness may pick up the extra stains, making
improper fishing angle slowly for better interpretation more challenging. Additional reagents can
technique. positioning. be trapped in wrinkles, folds, and air pockets, causing extra
artifacts on the slides
1.5 TISSUE STAININGPrepared by: IEESYA ATIFAH BINTI MOHD FAUZI
Haematoxylin & Eosin Staining
REAGENTS: Xylene, alcohol (Absolute, 95%), tap water, distilled Water, MATERIAL: Unstained Slide
Haematoxylin 3G (Sakura), Eosin
PRINCIPLE
APPARATUS: Reagent containers, slide racks, forceps, tissue paper, oven, hotplate HAEMATOXYLIN
PROCEDURE BLUE-PURPLE: Stains the nucleus of
the cells (chromatin within the
DEPARAFFINIZATION: To completely remove the wax. nucleus and nucleus membrane,
HYDRATION: 1. Decreasing alcohol concentration + water - To drain ribosomes, cytoplasmic regions
previous xylene and hydrate tissue. 2. Distilled water - Hydrate tissue. rich in RNA).
NUCLEAR STAINING: 1. Haematoxylin - Stains nucleic structure (DNA,
RNA). 2. Running tap water - Remove excess stain. EOSIN
BLUING: Distilled water - Uses alkalinity to change reddish-purple
haematoxylin to a blue/purple-blue colour. PINK: Stains basic elements
COUNTERSTAIN: 1. Eosin - Acidic counterstain to stain basic elements (erythrocytes, cytoplasm, muscle
(cytoplasm, muscle, collagen). 2. Distilled water - Remove excess eosin. and collagen). Stains in different
DEHYDRATION: Increasing alcohol % - To remove all traces of water. intensity of pink, according to
CLEARING: Xylene - Rinse tissue and render it completely transparent. different types of connective tissue.
Why hydrate tissue?
DEPARRAFINIZATION Deparrafinization Hydrated tissue makes it
of unstained slides. easier for aqueous reagents
The sectioned tissues were baked in the drying oven Hotplate: Pro, to readily penetrate the cells
at 59-60°C for 15-30 minutes to deparaffinize its HP-7 Lab Plus Series
paraffin parts and leave only the tissues adhered to and tissue elements.
the slides.
Xylene 3 min PROBLEMS SOLUTIONS
Xylene 3 min
Xylene 3 min
STAINING CYCLE Contaminated solutions The reagents were
and dyes - reagents filled replaced every 2-4 cycles
HYDRATION.
with excess wax and dyes. to ensure sufficient purity
1 min of reagents.
Absolute alcohol 1 min
1 min
Absolute alcohol 1 min
1 min
95% alcohol Overstain of haematoxylin. Solutions and
Wash in running tap water 5 min The blue-purple dye is too dyes were
5 min
Distilled water intense under replaced and the timings
NUCLEAR STAINING. 30 sec the microscope. of staining were timed
Haematoxylin 3G (Sakura)
correctly.
2 min
Wash in running tap water 10 sec
BLUING.
Tissue detached from slide The microtomb setting
Distilled water 10 sec
10 sec during staining due to thick was made sure on 3 µm
COUNTERSTAIN. 1 min
1 min tissue from sectioning. (for sectioning) to avoid
Eosin 1 min
too thick tissues.
Distilled water
1 min
DEHYDRATION. 2 min CONCLUSION AFTER
95% alcohol
95% alcohol The H&E procedure stains
Absolute alcohol the nucleus and cytoplasm BEFORE
Absolute alcohol contrasting colors to
Absolute alcohol
CLEARING. readily differentiate
Xylene cellular components.
Xylene
1.6 Prepared by: WAN ANISSA BINTI WAN MOHD ZAKI
SLIDE MOUNTING AND
LABELLING
PRINCIPLE: PROBLEM:
Present of air bubble after placing
A mounting medium is used the coverslip on the mounting
on the stained tissue slide to medium
adhere the coverslip to the
slide. SOLUTION:
A mounted stained tissue
slide with coverslip can The coverslip was gently pressed with an
preserve and support applicator stick to remove the bubbles. If too
sections for light microscopy. many bubbles were present, the slide was
placed in the xylene to remove the coverslip
and repeat the mounting procedure.
PROCEDURE:
1.Appropriate coverslip 2. Adequate mounting
was chose according to medium was placed on one
the size of the tissue edge of the slide.
section.
MATERIAL: 4. The slide was 3. The coverslip was
examined macroscopically gently lowered until it
H&E stained slide to ensure there are no touched the mounting
REAGENT: air bubbles. medium. Mounting medium
Mounting medium (CoverSeal-X) flowed upward due to
5. Label slide with the capillary attraction.
APPARATUS: following details:
6. The sticker
1.Slide rack Type of specimen labeled was
2. Coverslip Staining method placed at the
3.Applicator stick Student name frosted end of
4. Label sticker Student id the slide.
number
Date
RESULT: CONCLUSION:
H&E stained In slide mounting, it is important to
slide choose a good mounting medium in
order to have the best viewing and
preserving the slide during the
examination under the microscope.
Unlabeled or mislabeled of specimen
may lead to great clinical risk.
1.7Prepared by: WAN ANISSA BINTI WAN MOHD ZAKI
FROZEN SECTIONING
Frozen section technique is a rapid and useful histopathological
technique in preparing tissue for microscopic analysis. 5
PRINCIPLE:
The frozen section is the rapid
tissue section using cryostat in PROCEDURE: Embedding
cooling the tissue for immediate
report of tissue sample. 1. Chemicals/Reagents Preparation
95% alcohol (400 ml) was prepared.
1.380ml of Absolute Alcohol added to 20ml of distilled water.
SPECIMEN: 2. Tissue Sectioning
1. The cryostat was prepared by setting the temperature to
Fresh tissue sample
APPARATUS: -20°C. The forceps, microtome blade, brush and applicator
stick were placed in the appropriate tissue section in the
1.Clean glass slide cryostat.
2. Coverslip 2. A coplin jar containing 10% formalin was placed in the
3.Microtome blade oven at 60°C one hour before the specimen arrival. Before
4. Forcep using, it was removed from the oven.
5. Brush 3. The specimen was grossed.
6.Applicator stick 4. A glass slide was labelled with a type of specimen and
7.Coplin jar date.
5. The fresh tissue was embedded on a specimen disc with
EQUIPMENT:
1. Cryostat Tissue Freezing Medium and it was froze in the cryostat.
2. Oven Cryostat 6. The specimen disc was placed in the orientable specimen
CHEMICALS/REAGENTS: head.
1.Tissue Freezing Medium 7. The tissue was trimmed at 10µm thick until specimen is
2.10% Neutral Buffered Formalin exposed.
3.Hematoxylin 3G 8. Sectioning was performed at 5µm thick.
4. Eosin 9. The section was placed onto the labelled slide.
5. Xylene Immediately, it was placed in the pre-heated 10% formalin for
6.Alcohol (Absolute, 95%) about 1 minute.
7.Distilled water 10. Rapid H&E staining was performed. 8
8.Tap water REAGENT TIME
1. Absolute alcohol 10 dips
RESULT: 2. Running tap water Few dips
H & E stained 3. Hematoxylin 3G 1 minute
frozen section slide 4. Running tap water Few dips
5. Distilled water 15 seconds 9
6. Eosin 1 minute
PROBLEM: SOLUTION: 7. 95% Alcohol 10 dips
Introduced of ice The chuck covered 8. 95% Alcohol 10 dips
9. Absolute alcohol 10 dips
crystal artifact into thewith masking tape is
tissue. used at room 10. Absolute alcohol 10 dips
11. Xylene 10 dips
Shattering artifact in temperature on the 12. Xylene 10 dips
13. Xylene 10 dips
frozen section due to block face to warm
the overcooled block the tissue
CONCLUSION: 11. The slide was mounted and ready for microscopic
Frozen section is the best technique examination.
for rapid tissue interpretation. 12. The specimen disc was removed from the oriented specimen
- Tissue is preserved head and lei it melted at room temperature
- The storage of block is longer and can 13. The residual tissue was removed from the specimen disc and
be keep at room temperature. placed in the container containing 10% formalin
14. The cryostat was cleaned after used.
REFERENCES
1.FELDMAN, A. T., & WOLFE, D. (2014). TISSUE PROCESSING AND HEMATOXYLIN
AND EOSIN STAINING. METHODS IN MOLECULAR BIOLOGY (CLIFTON, N.J.),
1180, 31–43.
2.G. ROLLS. (N.D). STEPS TO BETTER GROSSING. RETRIEVED 2021, FROM
HTTPS://WWW.LEICABIOSYSTEMS.COM/KNOWLEDGE-PATHWAY/STEPS-TO-BETTER-
GROSSING/
3.GAO, X. H., LI, J., GONG, H. F., YU, G. Y., LIU, P., HAO, L. Q., LIU,
L. J., BAI, C. G., & ZHANG, W. (2020). COMPARISON OF FRESH FROZEN
TISSUE WITH FORMALIN-FIXED PARAFFIN-EMBEDDED TISSUE FOR MUTATION
ANALYSIS USING A MULTI-GENE PANEL IN PATIENTS WITH COLORECTAL CANCER.
FRONTIERS IN ONCOLOGY, 10. HTTPS://DOI.ORG/10.3389/FONC.2020.00310
4.GOLDBERG, A. (2018). A BRIEF HISTORY OF HISTOLOGY. RETRIEVED 2021,
FROM HTTPS://BLOG.LABTAG.COM/A-BRIEF-HISTORY-OF-HISTOLOGY/.
5.GUIDELINES FOR HAEMATOXYLIN AND EOSIN STAINING. (2001). NATIONAL
SOCIETY OF HISTOTECHNOLOGY. (BOWIE, M.D.).
6.MAHTAB UDDIN AHMED. (2016). STEPS OF TISSUE PROCESSING IN
HISTOPATHOLOGY LABORATORY,REVIEW REPORT. RETRIEVED 2021, FROM
HTTPS://WWW.RESEARCHGATE.NET/PUBLICATION/314950021_STEPS_OF_TISSUE_PR
OCESSING_IN_HISTOPATHOLOGY_LABORATORYREVIEW_REPORT
7.MOHAMED SLAOUI & L. FIETTE. (2011). HISTOPATHOLOGY PROCEDURES: FROM
TISSUE SAMPLING TO HISTOPATHOLOGICAL EVALUATION. RETRIEVED 2021, FROM
HTTPS://WWW.RESEARCHGATE.NET/PUBLICATION/47535516_HISTOPATHOLOGY_PROC
EDURES_FROM_TISSUE_SAMPLING_TO_HISTOPATHOLOGICAL_EVALUATION
8.PETERS, S. R. (2010). A PRACTICAL GUIDE TO FROZEN SECTION TECHNIQUE.
SPRINGER. RETRIEVED 2021, FROM
HTTPS://WWW.GARVAN.ORG.AU/RESEARCH/CAPABILITIES/HISTOPATHOLOGY/FILES/
A-PRACTICAL-GUIDE-TO-FROZEN-SECTION-TECHNIQUE.PDF.
9.R. THERESA, M. HARSHA, V. S. AMBERKAR. (2018). GROSSING OF TISSUE
SPECIMENS IN ORAL PATHOLOGY - ELEMENTAL GUIDELINES. RETRIEVED 2021,
FROM HTTPS://WWW.IJOHSJOURNAL.ORG/ARTICLE.ASP?ISSN=2231-
6027;YEAR=2018;VOLUME=8;ISSUE=2;SPAGE=63;EPAGE=67;AULAST=THERESA
10.ROLLS, G., & SAMPIAS, C. (2021). H&E STAINING OVERVIEW: A GUIDE TO
BEST PRACTICES. LEICA BIOSYSTEMS DIVISION OF LEICA MICROSYSTEMS INC.
11.SPENCER, L. T., & BANCROFT, J. D. (2012). MICROTOMY. BANCROFT'S
THEORY AND PRACTICE OF HISTOLOGICAL TECHNIQUES E-BOOK, 128-129.
12.SUVARNA, S. K., LAYTON, C., & BANCROFT, J. D. (2018). BANCROFT’S
THEORY AND PRACTICE OF HISTOLOGICAL TECHNIQUES. ELSEVIER
GEZONDHEIDSZORG.
13.WINSOR, L., & SLUYS, R. (2018). BASIC HISTOLOGICAL TECHNIQUES FOR
PLANARIANS. IN PLANARIAN REGENERATION (PP. 285-351). HUMANA PRESS,
NEW YORK, NY.
The words you are searching are inside this book. To get more targeted content, please make full-text search by clicking here.
1.NOR ALIAH SYAHIRAH BT MOHD HADZIR (2020498732)
2.NUR ALYAA QALISHA BINTI MOHAMMAD SAID (2020897946)
3.WAN ANISSA BINTI WAN MOHD ZAKI (2020498894)
4.IEESYA ATIFAH BINTI MOHD FAUZI (2020498516)
5.NUR MAISARAH BINTI MOHAMAD (2020853802)
Discover the best professional documents and content resources in AnyFlip Document Base.
Search
HS2413B _HISTOLOGICAL LAB REPORT GROUP 3
- 1 - 11
Pages: